Molecular Machines in Signalling Pathways
Background
Signaling networks are complex and robust, and they are based on macromolecular complexes with fine-grained structure-function modularity. At the cellular level, signaling networks are spatiotemporally regulated by post-translational modifications that render the systems highly dynamic. Our main research interest is focus on the characterization of protein complexes that are modulated by ubiquitination at the physiological and pathological levels. Ubiquitin is one of the most prevalent and elaborate post-translational modifications (PTMs) developed by eukaryotic cells. Over the last two decades, our and other studies have supported a paramount role of this PTM in the regulation of various fundamental processes such as endocytosis, DNA repair, cell migration and autophagy. Dysfunction of the ubiquitin network has been demonstrated, in particular, in the development and progression of cancer and neurodegeneration.
Ongoing research projects
Our line of research is multi-disciplinary, ranging from structure to function, with the final aim of reaching clinical intervention. We are using a variety of biochemical and biophysical techniques, including imaging, proteomics and crystallography, coupled with genetic tools (CRISPR) in mammals and Drosophila.
Our research spans over two major areas:
- The NEDD4 family of E3 enzymes as critical determinants of substrate and ubiquitin chain specificity, and coordinators of the downstream signaling pathways.
- The actin motor protein and ubiquitin binding receptor Myosin VI, with a special focus on its alternative splicing that is altered in cancer.
1. Targeting NEDD4 enzymes
E3s are the matchmakers of the ubiquitination process, able to modify selective substrates with specific kind of ubiquitin chains. By using the NEDD4 family as prototype and taking advantage of structural approaches, we shed light on the ubiquitination reaction catalyzed by the functional HECT domain of these E3 enzymes (Maspero et al, NSMB 2013). In addition, we characterized the regulation of the NEDD4 enzymes, identifying an auto-inhibitory interaction of the N-terminal C2 domain with the C-terminal HECT domain (Mari et al, Structure 2014) that is released upon tyrosine (Tyr) phosphorylation induced by receptor tyrosine kinases (RTKs) (Persaud et al, Sci Signal 2014). This molecular framework was instrumental to start studying the relevance of NEDD4 overexpression in cancer and to develop specific and selective inhibitors for this ubiquitin E3 enzyme.
Our current projects have the following aims:
- To define the complete mechanism of catalysis of the NEDD4 family members.
- To explore NEDD4 druggability, taking advantage of the structural knowledge we gained with a specific class of chemicals.
- To shed light on the functional role of the less characterized members of the family, i.e., HECW1 and HECW2, in human cell lines and Drosophila.
| Figure 1. Model of the ubiquitin chain assembly by the NEDD4 family of E3 ligases. Cartoon representation of the possible enzymatic events at the basis of substrate ubiquitination: blue: HECT N-lobe; green: HECT C-lobe; yellow: ubiquitin; dark red: E2; grey: WW domains; orange: substrate. First, the ubiquitin loaded onto the E2 (UbD) engage the HECT C-lobe, allowing the transthiolation reaction. Then, the C-lobe~Ub turns around the hinge region in order to face the substrate transferring the ubiquitin to it. At the second cycle, the C-lobe rotates in a similar way generating ubiquitin dimers. With the following steps, the N-lobe exosite (dashed circle on the N-lobe) helps to maintain the growing ubiquitin chain in close proximity and position the ubiquitin acceptor (UbA) in the correct orientation, limiting the C-lobe rotation and favoring enzyme processivity. Illustration by Cecilia Claudi, ceciliaclaudi88@gmail.com. |
2. Studying the impact of Myosin VI's alternative splicing
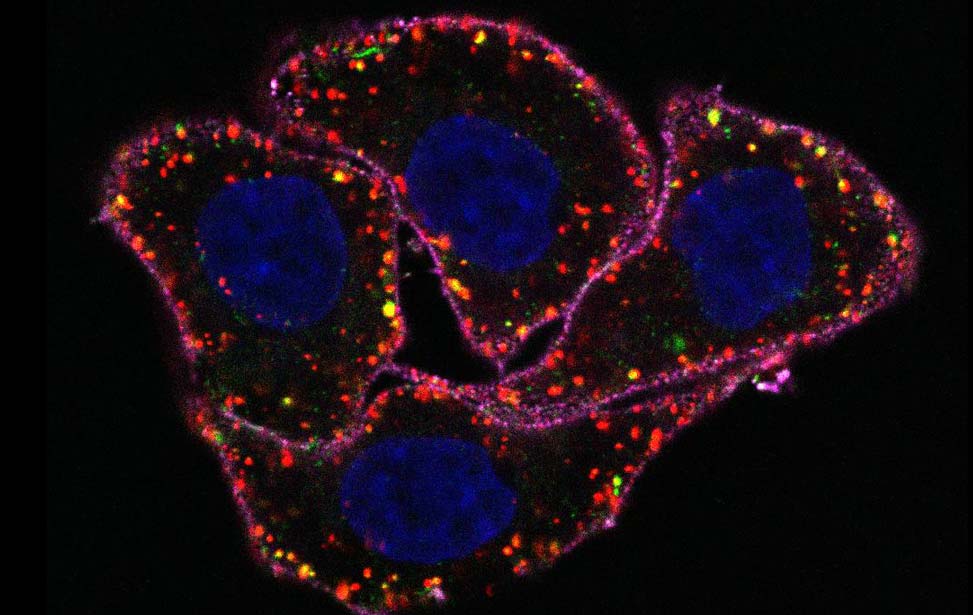
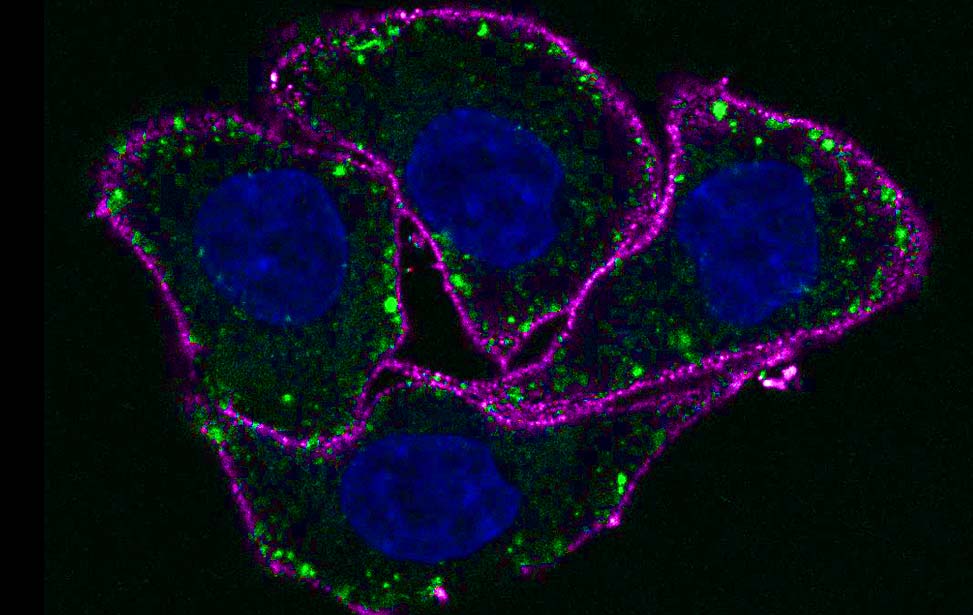
Among the 40 different myosins expressed in humans, myosin VI exhibits a unique directionality as it moves from the plus to the minus end of actin filaments. Myosin VI is implicated in endocytosis, exocytosis, autophagy and cell motility, and its dysfunction is associated with carcinogenesis. This protein combines both transport (e.g., vesicle movement) and anchoring (e.g., stereocilia maintenance) roles and is expressed as different isoforms. Our recent studies (Wollscheid et al, NSTMB, 2016; He et al, Cell Rep. 2016) demonstrated how myosin VI alternative splicing (AS) modulates its interactome, leading to different cellular functions (endocytosis for the long isoform and cell migration for the short one). Indeed, the different isoform structural architectures establish mutual exclusivity of myosin VI binding partners. Myosin VIlong is expressed upon cell polarization, whereas the short isoform is expressed in unpolarized, proliferating cells. Importantly, we found that myosin VI AS is aberrantly regulated in cancer, where exon skipping dictates cell addiction to myosin VIshort for tumor cell migration (Wollscheid et al, NSTMB, 2016).
The identification of myosin VIshort as a regulator of cancer cell migration provides an exciting starting point to understand and therapeutically exploit novel key events in pathological cell migration.
The primary scientific goals of our current studies are:
- To characterize the functional role of myosin VI in cancer cell migration.
- To characterize the switch between myosin VI isoforms that occurs during the establishment of cell polarity.
- To unravel the function of myosin VI as ubiquitin receptor.
| Figure 2. Alternative splicing region is the critical determinant of the myosin VI diverse functions. A. Establishment and regulation of apico-basal polarity is a cornerstone of normal epithelial tissues and relies on spatially delimited endocytic events of adhesion molecules. Our published data identified clathrin as specific interactor of myosin VIlong and the identified myosin VI-clathrin axis may well contribute to maintain the integrity of normal cell- and tissue-architecture by regulating endocytic events occurring at the apical surface of polarized cells. B. A hallmark of transformation and tumorigenesis is the epithelial-to-mesenchymal transition (EMT) where proteins found at cell-cell junctions reorganize at the invasive edge of the cell to promote motility and invasion. Moreover, partial EMT, resulting in a hybrid epithelial/mesenchymal phenotype with retention of E-cadherin, appears to be essential for dissemination of cohesive cohorts of cells. How myosin VIshort impacts in cancer cell dissemination is still unknown. Myosin VIshort could anchor or deliver critical players at the tip of the lamellipodium of migrating cells (upper panel) or may confer mechanical resistance to tension increase across junctions of migrating cluster of cells by anchoring E-cadherin to the cytoskeleton (lower panel). On the right panels, models of the two isoforms. In orange, the alternative spliced region that is included exclusively in myosin VIlong. Main isoform-specific interactors (clathrin and optineurin, respectively) are indicated. Illustration by Cecilia Claudi, ceciliaclaudi88@gmail.com. |