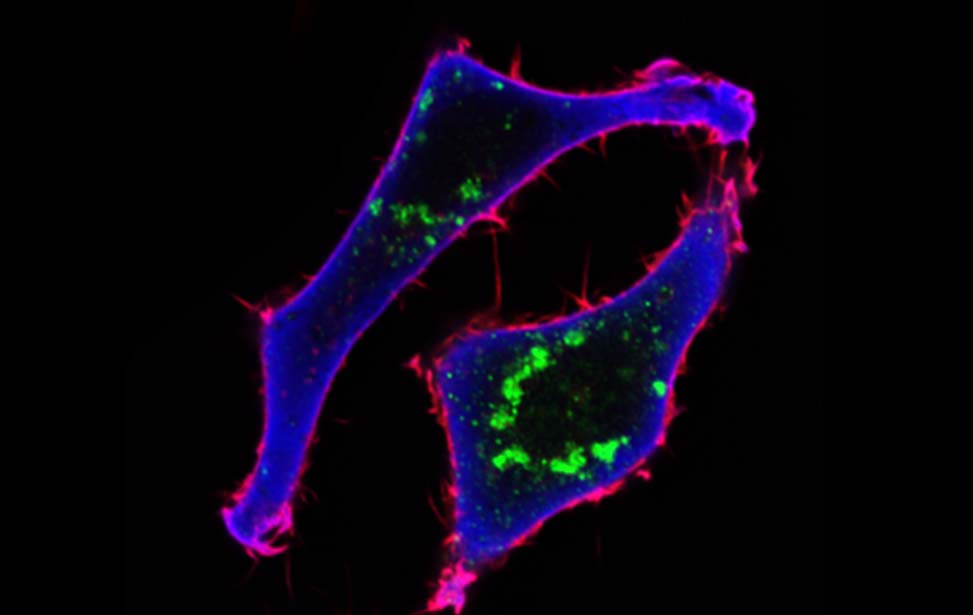
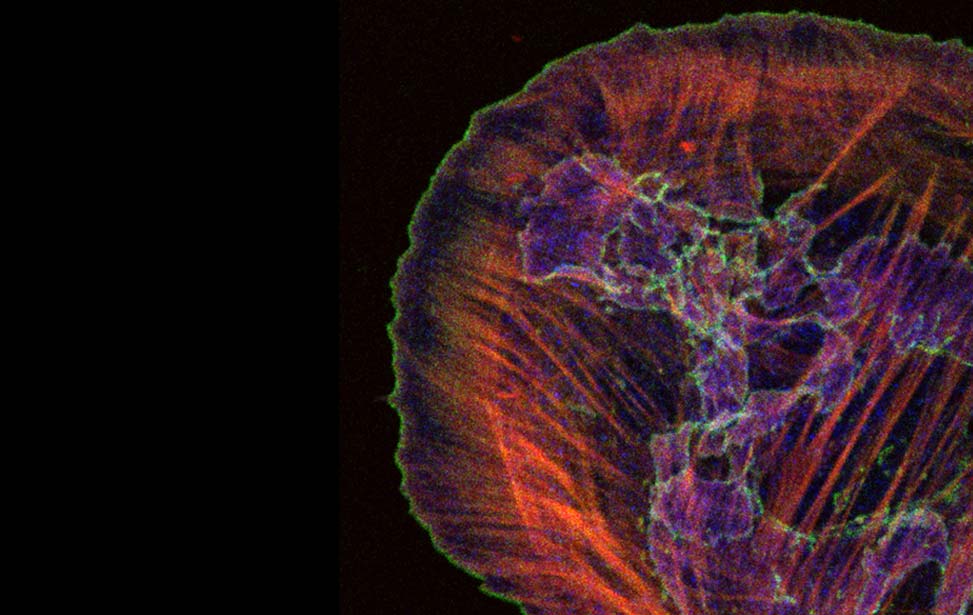
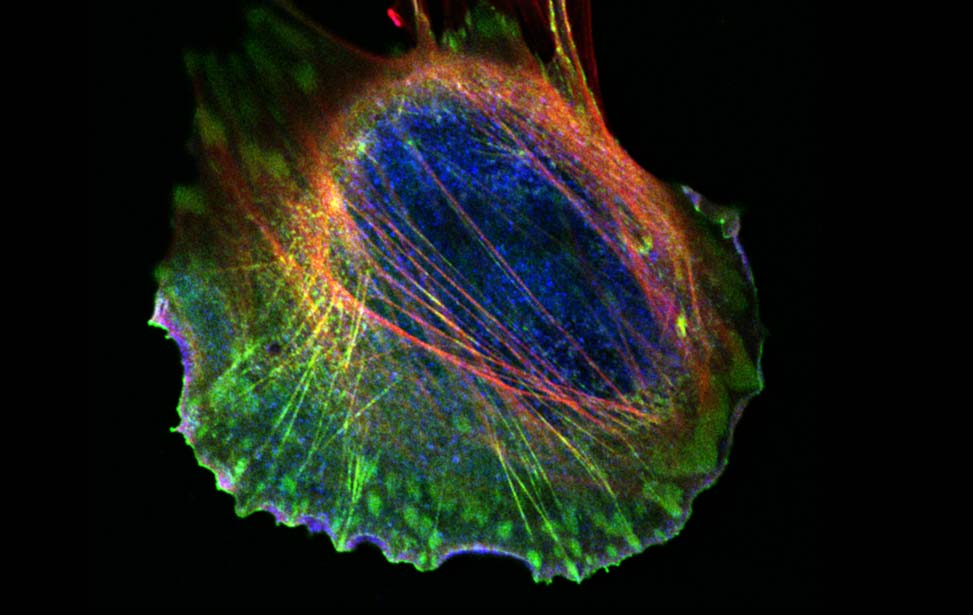
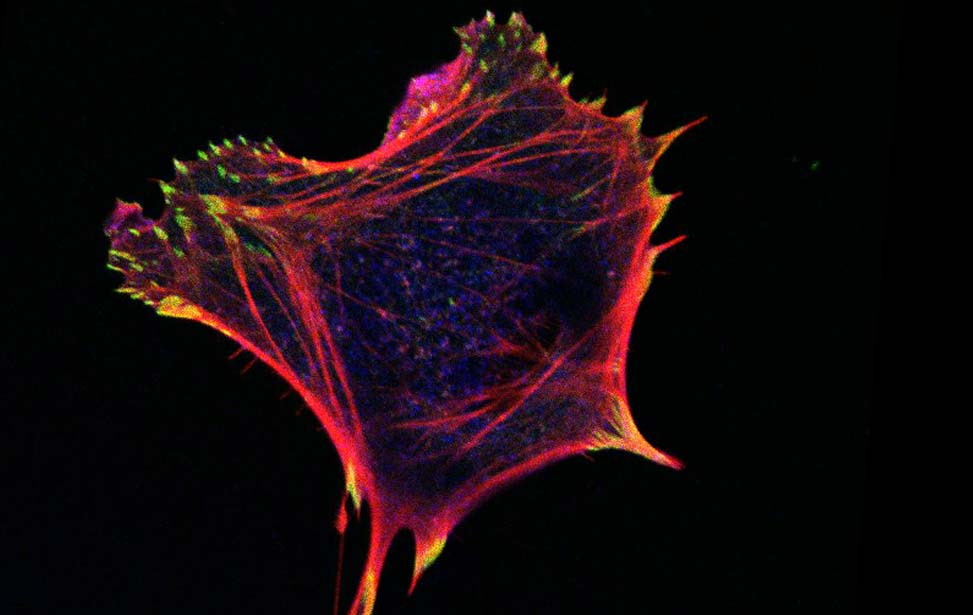
Mechanisms of Tumor Cell Migration
Research project
The pathophysiology of tissue fluidification
The early steps of carcinogenesis are the processes by which locally confined epithelial malignancy progressively evolves into invasive cancer. These processes are associated with the acquisition of cell motility and disrupted cell and tissue organization and dynamics, fostered by a mechanical shift from a solid-like to a liquid-like state (Giavazzi et al., 2018; Kai et al., 2016; Malinverno et al., 2017; Oswald et al., 2017; Palamidessi et al., 2019).
Top panels: analysis of the dynamic evolution of mammary epithelial monolayers, which at a critical density undergo a fluid-to-solid phase transition
Middle panels: Rab5A-induces a transition to a flocking fluid state that results in persistent rotation of 3D tumoral spheroids
Bottom panel: shape alterations in human ductal DCIS underlie the transition from a solid, jammed (left) to a fluid, unjammed State (right)
Like inert soft materials, such as foams and colloids, normal and malignant epithelial tissues can undergo a solid-to-fluid phase transition, which enables them to flow and their constituents to rearrange, while maintaining overall mechanical integrity. During organ development, normal epithelial tissues frequently evolve into solid or jammed masses that are densely packed with cells. This is thought to ensure the proper development of barrier properties and elasticity in epithelial tissue and act as a tumor suppressive mechanism for the growth and expansion of individual oncogenic variants. To repair a wound, regenerate, or become malignant, a certain degree of fluidity is required for a tissue to be able to proliferate, migrate and disseminate. A recently discovered process to account for this behavior is cellular unjamming, a phase transition characterized by collective and cooperative cellular motion akin to fluid flow, and remarkably similar to the motion of an ensemble of living collective animals, like herds of sheep or school of fish (Ilina et al., 2020; Malinverno et al., 2017; Mitchel et al., 2020; Oswald et al., 2017; Palamidessi et al., 2019; Park et al., 2016; Park et al., 2015; Tetley et al., 2019).
A remarkable case where tissue levels phase transition is pivotal is in the progression of breast Ductal adenoCarcinoma In Situ (DCIS), a precursor of invasive breast cancer, into Invasive Ductal Carcinoma (IDC). Recently, we have characterized an endocytic-dependent, mechanically driven, collective motility strategy of cell dissemination by solid-to-liquid–or unjamming-transition through flocking, which is involved in the progression from DCIS to IDC in human breast cancer (Atia et al., 2018; Kuriyama et al., 2014; Malinverno et al., 2017; Palamidessi et al., 2019; Park et al., 2015). Specifically, we have shown that RAB5A, a master regulator of endocytosis whose expression is elevated in aggressive human breast cancer and correlates with reduced relapse-free survival of patients (Frittoli et al., 2014), is sufficient to reawaken via tissue fluidification the collective motility of kinetically arrested DCIS. RAB5A can also promote the persistent angular collective motion of DCIS spheroids, leading to individual and collective invasion in 3D matrices and murine models. This is also accompanied by a wholesale transcriptional rewiring toward a cGAS/STING-dependent innate immune response that dramatically affects the fate and behavior of tumor collectives (Frittoli et al., 2022).
Altogether this evidence indicates that unjamming via flocking is an alternative or better complementary gateway to cell migration with respect to the more canonical epithelial to mesenchymal transition. Like EMT, also unjamming and fluidification transcriptional rewires tissue enabling the emergence of tumor disseminating properties, including chemoresistance. We posit, however, that these changes may also trigger an anti-tumor immune response and unveil immuno-based cancer vulnerabilities.
There are several, overarching unanswered issues on the mechanisms and pathophysiological role of epithelial tissue fluidification during the progression of breast carcinoma, which we aim to address through a combination of physical, biophysical, and cancer cell biology approaches.
Specific questions we are currently addressing
- What are the molecular and physical mechanisms controlling tissue fluidification in epithelial cells and breast tumor models of DCIS?
- How the mechanical perturbations associated with unjamming by impacting the acquisition of heritable changes influence cell phenotypes, fate, and tumor ecology (tumor cell interaction with immune cells). We will specifically focus on unexpected cellular processes and molecular mechanisms underlying tissue fluidification-dependent genetic rewiring toward a cGAS-STING-mediated innate immunity response by studying the role of mitochondria dynamics, morphology, and integrity during unjamming-via-flocking.
- What are the pathobiological consequence of tissue fluidification by determining whether tissue fluidization can turn immunological cold lesions into hot ones using syngeneic mice models and human specimens
Shape & CellFate
In addition, parallel to this program, we have recently been awarded an ERC-synergy-grant, ShapinCellFate, together with Matthieu Piel (CNRS, Paris, France), Raphaël Voituriez (CNRS, Paris, France), and Ana-Maria Lennon-Duménil (INSERM, Paris, France). The overarching aim of the proposal is to relate the history of Cell Shape Changes to Cell Behavior and Fate in dendritic and breast cancer cells. Briefly: Cells are often depicted as irregular spherical objects - the shape they adopt in suspension. However, the packed environment of tissues alters this simple shape, causing large cell deformations. This occurs during normal tissue growth and is even more pronounced upon tissue overgrowth, as in the case of solid tumors. Cell shape changes frequently occur in migratory cells, such as immune cells that patrol the organism within interstitial tissues, and cancer metastases that escape from the primary tumor to invade healthy tissues. In all cases, cells adapt and survive even very large deformations. The mechanisms underlying such response and the long-term consequences that repeated cell shape changes have on physiology and pathology remain largely unknown. We have observed that changes in the shape of cells and organelle(s) induce reversible and irreversible modifications in their behavior and function(s). We hypothesize that cells use such mechanisms to integrate the successive deformations of distinct amplitudes and durations that they experience during their lifetime. This implies the existence of “shape-induced memory effects” that not only encode the geometrical and mechanical history of the cell but also dictate its fate. Here, we propose to tackle the molecular mechanisms and physical principles accounting for shape-induced memory effects and to evaluate their impact on immunity and cancer. We will focus on two cell types that undergo large shape changes in vivo, and communicate to establish cancer immunity: (1) dendritic cells, which initiate adaptive immune responses, and (2) cancer cells derived from mammary epithelia. Our project will reveal whether boundary conditions imposed by physical confinement are overarching determinants of cellular behaviors at different spatial and temporal scales, and may further establish novel clinical paths for a holistic understanding of early malignancies and their recognition by the immune system.